
Neuralink’s recent breakthrough marks a monumental stride, in the ever-evolving intersection of technology, medicine, and innovation. Founded by Elon Musk in 2017, Neuralink has been at the forefront of developing brain-computer interfaces (BCIs) with the potential to revolutionize how humans interact with technology. The company’s first successful implantation of a brain chip in a human patient—who has since fully recovered and can now control a computer mouse through thought alone—ushers in a new era of medical and technological integration.
This achievement, as announced by Musk, underscores the rapid advancements in neural technology and its applications. The ability for a human to interact with digital interfaces through mere thought was once the realm of science fiction. Today, it represents the tangible progress Neuralink has made towards its ambitious goals. The initial focus on individuals with quadriplegia due to cervical spinal cord injury or amyotrophic lateral sclerosis (ALS) demonstrates Neuralink’s commitment to addressing some of the most challenging medical conditions through innovative solutions.
Neuralink’s technology utilizes a robot to surgically implant a BCI into specific brain regions that control movement intention. The primary aim is to enable users to navigate digital interfaces—such as cursors or keyboards—using only their thoughts. This not only promises to restore independence and improve the quality of life for individuals with severe motor impairments but also opens the door to further applications in treating a wide array of neurological conditions.
Musk’s broader vision for Neuralink extends beyond immediate medical applications. He envisions a future where BCIs can address a range of conditions, including obesity, autism, depression, and schizophrenia, through rapid and minimally invasive surgical implantation of chip devices. Furthermore, Musk contemplates the long-term possibility of merging human intelligence with artificial intelligence, enhancing sensory capabilities, and even overcoming hearing and vision loss. These goals highlight the dual focus of Neuralink: to provide immediate benefits through assistive technology while exploring the potential for human enhancement and expanded cognitive capabilities.
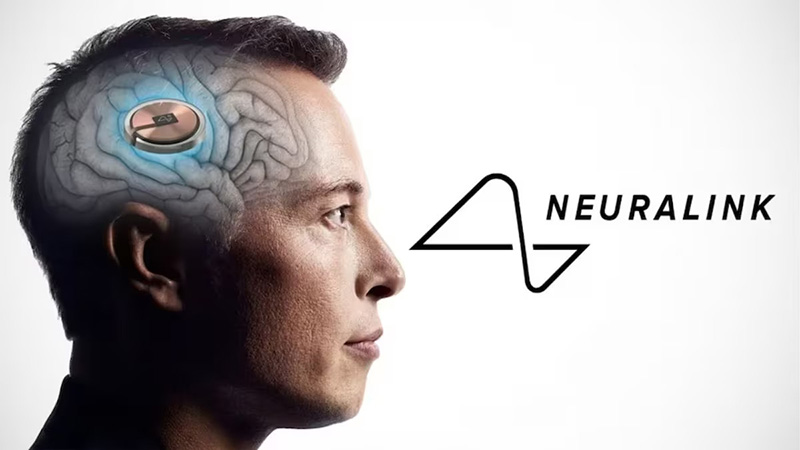
However, Neuralink’s journey is not without challenges. The company, valued at about $5 billion, has faced scrutiny over its safety protocols. Ensuring the safety and efficacy of such invasive technology is paramount, given the potential risks involved in brain surgery and long-term implantation. Regulatory approval processes for human trials, such as the one Neuralink has embarked on, are rigorous and designed to protect participants while assessing the technology’s potential benefits and risks.
The timeline for Neuralink’s technology to move from experimental trials to commercial availability is measured in years, reflecting the cautious approach required in medical innovation. Initial studies typically involve a small cohort of patients and last up to a year, focusing on feasibility and safety. Subsequent phases will test the technology’s effectiveness and broader applicability before it can be considered for commercial use. This process, likely spanning five to ten years, is crucial for ensuring that Neuralink’s BCIs are both safe and beneficial for a wider population.
Neuralink’s work represents a convergence of business innovation, medical advancement, and technological exploration. As BCIs move from theory to practice, they hold the promise of transforming lives by restoring lost functions and expanding human capabilities. The success of Neuralink’s first human implant trial is a significant milestone, signaling the potential of BCIs to redefine the boundaries of human-machine interaction.
Yet, the ethical, social, and regulatory implications of such technology necessitate careful consideration. As Neuralink progresses, it will need to navigate these challenges transparently and responsibly, ensuring that the benefits of BCIs are accessible and equitable. The collaboration between engineers, neuroscientists, clinicians, and ethicists will be key to realizing the full potential of this technology while safeguarding human values and dignity.
In conclusion, Neuralink’s pioneering efforts in developing brain-computer interfaces highlight the remarkable potential of integrating technology with the human brain to address complex medical challenges and enhance human capabilities. As this field advances, it will undoubtedly raise important questions about the future of human interaction with technology, the nature of consciousness, and the ethical dimensions of technological enhancement. Neuralink’s journey is not just about pushing the boundaries of what is technologically possible but also about envisioning a future where technology and humanity converge in ways that enhance life and open new realms of possibility.
Prof. Dr. Prahlada N. B
01 March 2024
Chitradurga.
















A thought provoking summary!
ReplyYes Prahlada Sir,
Neuralink, a pioneering company founded by Elon Musk, is working on developing advanced brain-computer interfaces. These interfaces aim to establish a direct communication pathway between the brain and digital devices, allowing humans to interact with technology in unprecedented ways.
The potential applications of such technology are vast as told by you, ranging from treating neurological disorders to enhancing human cognitive abilities.
As research and development progress, Neuralink's efforts may pave the way for a more integrated and seamless relationship between humans and technology.
Reply